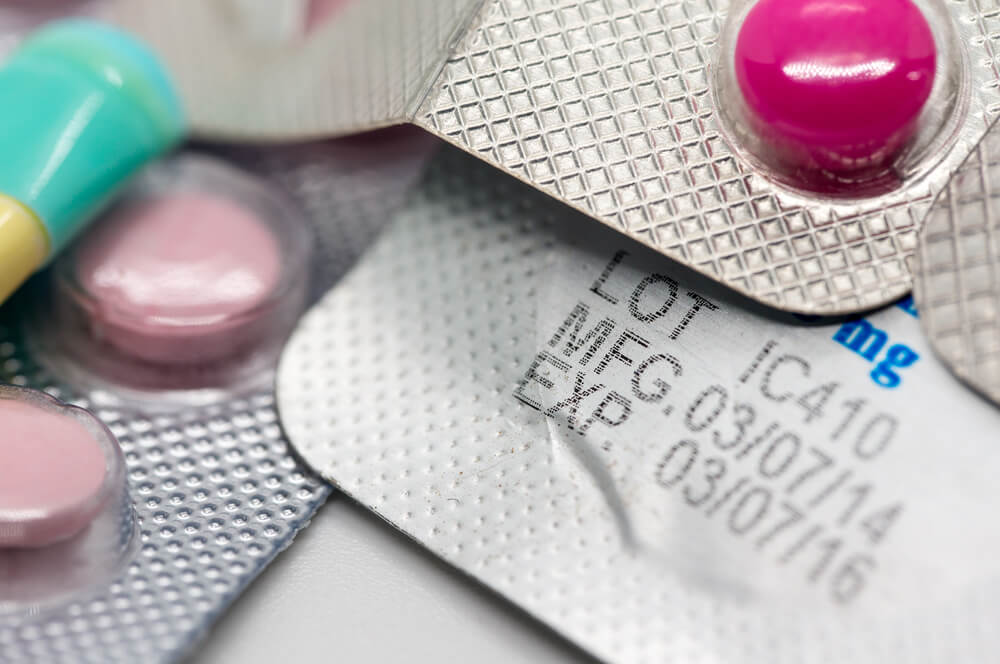
The expiration date is the last day that the manufacturer guarantees the safety of a medication. Drug expiration dates exist on medication labels, prescription, over-the-counter and dietary (herbal) supplements. For legal and liability reasons, manufacturers will not make recommendations about the stability of drugs past the original expiration date. However, for most drugs, it's just an arbitrary date, usually 2 or 3 years out, that the manufacturer selects to test drug stability. In all actuality, the stability of the drug may be much longer, but no one tests it. The expiration date of a drug is estimated using stability testing under good manufacturing practices as determined by the FDA. Drug products marketed in the US typically have an expiration date that extends from 1 to 5 years from the time of manufacturer. Once the original container is opened, either by the patient or the health care provider who will dispense the drug, that original expiration date on the container can no longer be relied upon. However, the actual shelf life of the drug may be much longer as stability studies have shown. At the pharmacy, "beyond-use" dates are often put on the prescription bottle label given to the patient. These dates often say "do not use after..." or "discard after..." and are required by the Board of Pharmacy in many states. These dates are typically one year from the date of fill. But why would these expiration dates be different? According to the manufacturer, the stability of a drug cannot be guaranteed once the original bottle is opened. Heat, humidity, light, and other storage factors can affect stability. Plus, pharmacies, both retail and hospital, nursing homes, and consumers toss away billions of dollars of medications each year based on stamped expiration dates on stock bottles. The United States Pharmacopeia (USP), the body that sets the standards for pharmaceutical quality in the U.S., recommends using "beyond use" dates. The "beyond use" date would never be later than the expiration date on the manufacturer's bottle. However, the expiration date on the prescription bottle from the pharmacy is usually one year from the date it was filled; again, another arbitrary date. There's really no way to know unless drugs are tested, but here are some common-sense measures: Insulin is used to control blood sugar in diabetes and may be susceptible to degradation after its expiration date. Oral nitroglycerin (NTG), a medication used for angina (chest pain), may lose its potency quickly once the medication bottle is opened. Vaccines, biologicals or blood products could also be subject to quick degradation once the expiration date is reached. Tetracycline may produce a toxic metabolite, but this controversial among researchers. Medicines that looks old: powdery or crumbling medicine, drugs with a strong smell, or dried up medicine (as in the case of or ointments or creams) should be discarded. Proper storage of medications may help to extend their potency. The bathroom and medicine cabinet are not ideal places to store medications due to heat and humidity. Similarly, medications should not be left in a hot car or glovebox, or in freezing weather. Medications remain most stable in dry, cool spaces away from light. Keep the prescription bottle caps tightly closed and always keep medications out of reach of children and pets. References: 1. American Society of Health System Pharmacists (ASHP.org). Q&A on Proposed USP Chapter 797 Revisions with E. Clyde Buchanan. 2. Drug Expiration Dates - Do They Mean Anything? Harvard Health Publishing. Harvard Medical School. August 13, 2017. Accessed May 29, 2018 at https://www.health.harvard.edu/staying-healthy/drug-expiration-dates-do-they-mean-anything 3. American Medical Association. "Pharmaceutical Expiration Dates." Report 1 of the Council on Scientific Affairs (A-01). July 25, 2001. 4. Lyon RC, Taylor JS, Porter DA, et al. Stability profiles of drug products extended beyond labeled expiration dates. J Pharm Sci 2006;95:1549-60. Accessed May 29, 2018 at https://misuse.ncbi.nlm.nih.gov/error/abuse.shtml By Garibli A.
If this page is in your subscriptions, then it will be removed. You will not see this page. If you want to unblock a user, go to the settings, the list of blocked users and click unblock